Quality Assurance vs Quality Control: Differentiating Proactive and Reactive Approaches to Quality
Umang Chauhan
Mar 15, 2024
Introduction
Delivering high-quality products and services is paramount for success in modern business. Two critical components of quality management are Quality Assurance (QA) and Quality Control (QC). While often used interchangeably, QA and QC are distinct concepts with specific roles in ensuring excellence.
This comprehensive guide will delve into the fundamentals of Quality Assurance and Quality Control, their contrasts, industry-specific applications, and how they synergistically uphold quality standards that delight customers and drive business growth.
Defining the Fundamentals
Quality Assurance
Quality Assurance (QA) is a proactive, process-oriented approach that prevents defects and non-conformances throughout the product or service lifecycle. QA establishes a planned and systematic set of activities to instill confidence that quality requirements will be fulfilled consistently.
The core tenets of Quality Assurance include:
- Establishing quality objectives and standards
- Developing processes, procedures, and best practices
- Identifying potential risks and implementing preventive measures
- Continuously monitoring and improving the quality system
QA aims to build quality into the product or service from the onset, reducing the likelihood of defects and customer dissatisfaction.
Quality Control
Quality Control (QC) is a reactive, product-oriented approach that focuses on identifying and correcting defects or non-conformances in finished products or services before they reach the customer. QC involves a series of specific tests, inspections, and measurements to verify that the output meets the defined quality criteria.
The primary goals of Quality Control include:
- Detecting and eliminating defects or non-conformances
- Ensuring that products or services meet specifications
- Verifying the effectiveness of the quality system
- Providing feedback for process improvements
QC is the final checkpoint to prevent defective or substandard products or services from reaching the customer.
Quality Assurance and Quality Control in Depth
The Process of Quality Assurance
Quality Assurance is a continuous process that spans the entire product or service lifecycle. It begins with understanding and translating customer requirements into specific, measurable quality objectives. QA teams then develop quality plans outlining the processes, procedures, and resources needed to achieve these objectives.
Key Quality Assurance activities include:
- Process mapping and risk analysis
- Developing standard operating procedures (SOPs)
- Conducting training and competency assessments
- Performing quality audits and management reviews
- Implementing corrective and preventive actions (CAPA)
Organizations investing in quality management systems and quality assurance practices can expect significant financial benefits. The American Society for Quality (ASQ) conducted a study that revealed that organizations implementing effective quality management systems can expect a 3-4% increase in revenue. This increase is attributed to improved customer satisfaction, reduced costs, and increased efficiency. Additionally, the study found that organizations can achieve a 10-20% reduction in the cost of poor quality. This reduction results from fewer defects, rework, and warranty claims.
The benefits of investing in quality management systems and quality assurance practices are well-documented. In addition to financial benefits, organizations experience improved customer satisfaction, increased employee morale, and enhanced competitiveness.
Here are some specific examples of how quality management systems and quality assurance practices can lead to financial benefits:
- Increased sales: When customers are satisfied with a product or service, they are more likely to repeat purchases and recommend the company to others. This can lead to increased sales and revenue.
- Reduced costs: Quality management systems can help organizations reduce costs by identifying and eliminating waste. This can be achieved through improved processes, reduced defects, and better inventory management.
- Improved efficiency: Quality management systems can help organizations improve efficiency by streamlining processes and reducing rework. This can lead to increased productivity and lower costs.
- Reduced warranty claims: Quality management systems can help organizations reduce warranty claims by ensuring that products are manufactured to meet customer requirements. This can lead to significant cost savings.
- Increased profitability: The above benefits can lead to increased profitability for organizations that invest in quality management systems and quality assurance practices.
The Techniques of Quality Control
Quality Control employs various techniques to verify that products or services meet the specified requirements. These techniques can be classified into two main categories: inspection and testing.
Inspection techniques involve visual examination, measurement, or functional checks to identify defects or non-conformances. Common inspection methods include:
- Dimensional measurements
- Visual inspections for defects
- Functional tests
- Statistical sampling plans
Testing techniques involve subjecting the product or service to specific conditions or inputs to verify its performance, reliability, or safety. Examples of testing methods include:
- Destructive testing: This type of testing involves breaking a product or component to determine its limits and weaknesses. It is often used to evaluate a product's safety and durability. Examples of destructive testing include crash-testing cars, drop-testing phones, and tensile-testing materials.
- Non-destructive testing (NDT): NDT is a method of evaluating the condition of a product or component without causing any damage. It is often used to detect defects or flaws in materials or structures. Examples of NDT include X-ray inspection, ultrasonic testing, and magnetic particle inspection.
- Accelerated life testing: This type of testing involves subjecting a product or component to stress conditions that are more severe than normal operating conditions. It is used to assess a product's long-term durability and reliability. Examples of accelerated life testing include temperature cycling, humidity testing, and vibration testing.
- Stress testing involves applying a load or stress to a product or component to determine its ability to withstand those conditions. It is often used to evaluate a product's performance under extreme conditions. Stress testing includes load testing, pressure testing, and electrical stress testing.
The choice of QC techniques depends on the nature of the product or service, the associated risks, and the applicable industry standards or regulations.
Quality Assurance vs Quality Control in Practice

Contrasting QA and QC
While QA and QC aim to ensure quality, they differ in focus, timing, and approach.
Quality Assurance:
- Focuses on processes and systems
- Proactive and preventive approach
- Spans the entire product or service lifecycle
- Responsibility of all employees
- Emphasizes continuous improvement
Quality Control:
- Focuses on products or services
- Reactive and corrective approach
- Occurs at specific stages, typically at the end of the process
- Responsibility of dedicated QC inspectors or testers
- Emphasizes defect detection and elimination
Examples and Case Studies
To illustrate the practical application of QA and QC, consider the following examples:
Software Development:
- QA: Establishing coding standards, conducting code reviews, and performing automated testing throughout development.
- QC: Executing manual and automated tests on the final software build to identify and fix bugs before release.
Manufacturing:
- QA: Implementing statistical process control (SPC) to monitor and adjust the manufacturing process in real time.
- QC: Conducting final inspections and functional tests on finished products to ensure they meet specifications.
Service Industry:
- QA: Developing standard operating procedures (SOPs) and training employees to deliver consistent, high-quality service.
- QC: Conducting customer satisfaction surveys and mystery shopping to evaluate service quality and identify areas for improvement.
Industry-Specific Quality Practices
Construction Industry
QA and QC are critical in ensuring building and infrastructure safety, reliability, and performance in the construction industry.
Quality Assurance in construction involves:
- Developing project quality plans
- Conducting design reviews and constructability analyses
- Implementing quality control inspection and testing plans
- Performing audits and management reviews
Quality Control in construction focuses on the following:
- Inspecting materials and equipment
- Verifying construction work against drawings and specifications
- Conducting field tests and commissioning activities
- Documenting non-conformances and corrective actions
According to a study by the Construction Industry Institute, projects with effective QA/QC programs experience 2.6% fewer rework hours, 6.0% lower total project costs, and 15.2% shorter schedule durations compared to projects with ineffective quality programs.
Software Testing
QA and QC are integral to the testing process in software development, ensuring that software products meet functional and non-functional requirements.
Quality Assurance in software testing involves:
- Developing test plans and test case specifications
- Conducting static testing (reviews, walkthroughs, and inspections)
- Implementing test automation frameworks
- Performing root cause analysis and implementing preventive measures
Quality Control in software testing focuses on the following:
- Executing manual and automated tests
- Reporting and tracking defects
- Verifying bug fixes and conducting regression testing
- Performing user acceptance testing (UAT)
National Institute of Standards and Technology (NIST) found that software bugs cost the U.S. economy $59.5 billion annually. Software users bear 50% of the cost, and software developers and vendors bear 50%. Implementing effective QA and QC practices can significantly reduce these costs.
Food and Pharmaceutical Industries
QA and QC are critical for ensuring product safety, efficacy, and compliance with stringent regulatory requirements in the food and pharmaceutical industries.
Quality Assurance in these industries involves:
- Implementing Good Manufacturing Practices (GMP)
- Developing and validating production processes
- Conducting supplier audits and material qualifications
- Performing risk assessments and implementing risk controls
Quality Control in these industries focuses on:
- Conducting raw material and finished product testing
- Performing in-process checks and environmental monitoring
- Investigating and reporting deviations and non-conformances
- Ensuring proper documentation and record-keeping
Investing in quality assurance (QA) and quality control (QC) systems is a wise financial decision for pharmaceutical companies. According to the World Health Organization (WHO), pharmaceutical companies can expect a 5:1 return on investment (ROI) by implementing robust QA and QC systems. This ROI is realized through several tangible benefits, including:
- Reduced Product Recalls: QA and QC systems help identify and prevent product defects before they reach the market. This reduces the risk of costly product recalls, damaging a company's reputation and leading to financial losses.
- Improved Patient Safety: QA and QC systems ensure that pharmaceutical products meet the highest safety and efficacy standards. This helps protect patients from harm and fosters trust in the company's products.
- Enhanced Brand Reputation: A strong commitment to quality builds trust with healthcare professionals, consumers, and regulators. This can increase sales, market share, and overall brand value.
- Increased Efficiency and Productivity: QA and QC systems help streamline production processes and reduce waste. This can improve efficiency, productivity, and profitability.
- Compliance with Regulatory Requirements: Robust QA and QC systems help pharmaceutical companies comply with regulatory requirements, such as those set by the FDA and EMA. This reduces the risk of legal penalties and ensures smooth market access.
- Improved Communication and Collaboration: QA and QC systems promote effective communication and collaboration between different departments within the pharmaceutical company. This can help identify and resolve issues promptly, improving decision-making and overall performance.
Investing in QA and QC systems is a strategic imperative in today's competitive pharmaceutical market. Pharmaceutical companies prioritizing quality will be well-positioned to succeed in the long term.
Quality Assurance Plans vs Quality Control Plans
Quality Assurance and Quality Control Plans are essential documents that outline the specific activities, resources, and responsibilities for ensuring product or service quality.
A Quality Assurance Plan typically includes:
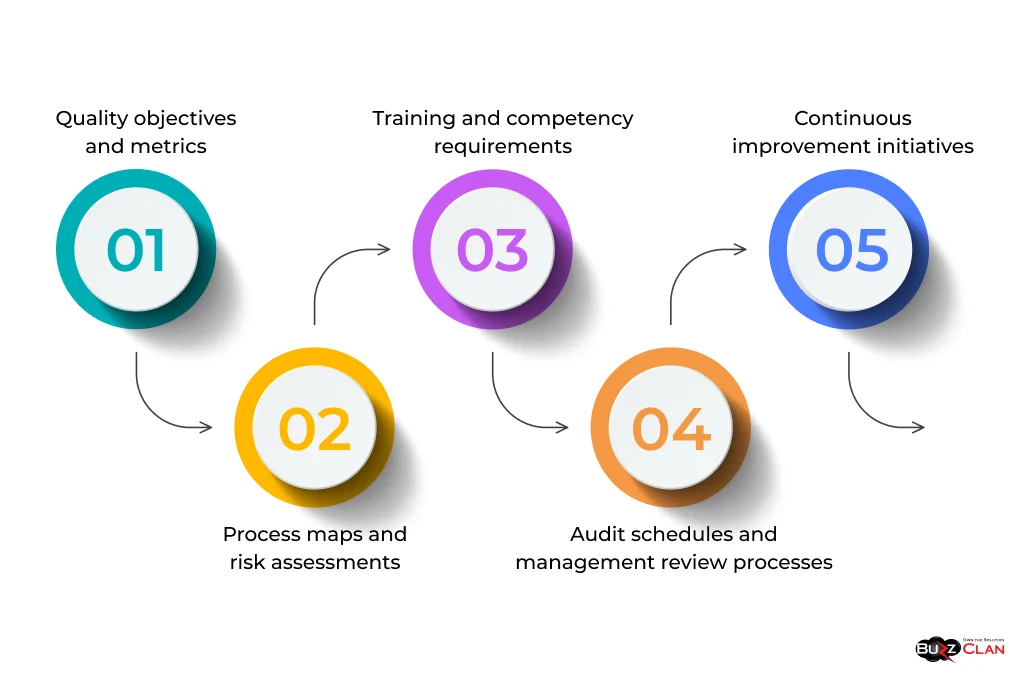
- Quality objectives and metrics
- Process maps and risk assessments
- Training and competency requirements
- Audit schedules and management review processes
- Continuous improvement initiatives
A Quality Control Plan typically includes:
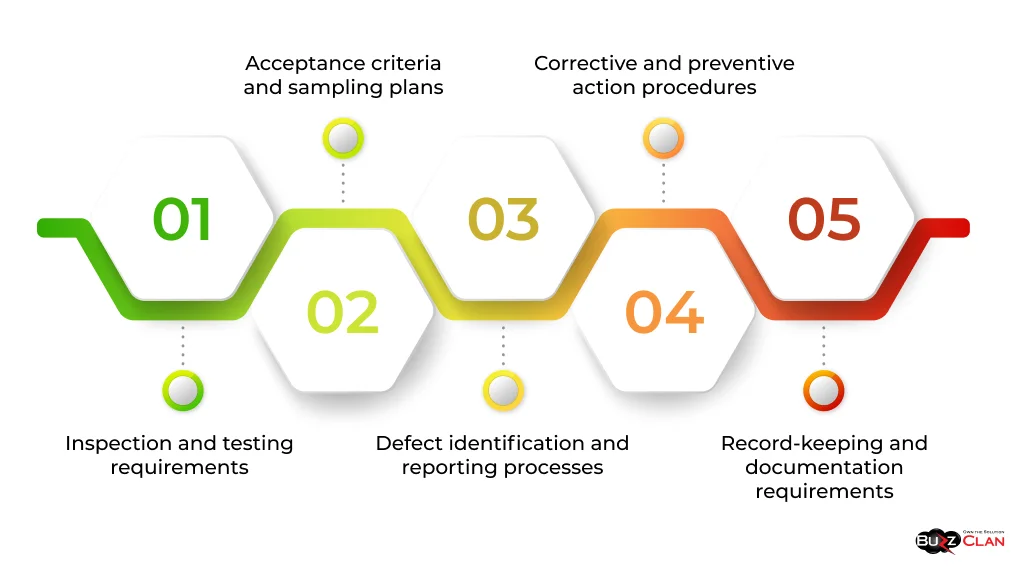
- Inspection and testing requirements
- Acceptance criteria and sampling plans
- Defect identification and reporting processes
- Corrective and preventive action procedures
- Record-keeping and documentation requirements
Quality Assurance vs Quality Control Documentation and Reporting
Accurate and timely documentation is crucial for demonstrating compliance, traceability, and continuous improvement in quality management systems.
Key quality documents include:
- Standard operating procedures (SOPs)
- Work instructions and checklists
- Inspection and test reports
- Non-conformance and corrective action reports
- Training records and competency assessments
Effective documentation ensures that quality activities are performed consistently, problems are identified and resolved promptly, and lessons learned are captured for future reference.
Integrating Quality Assurance and Quality Control
While QA and QC have distinct roles, they are most effective when integrated and aligned toward continuous quality improvement. Some ways to create synergy between QA and QC include:
- Fostering collaboration and communication between QA and QC teams
- Sharing data and insights from QC activities to inform QA process improvements
- Involving QC teams in the development of quality plans and procedures
- Conducting joint root cause analysis and problem-solving sessions
By working together, QA and QC can create a more robust and effective quality management system that prevents defects, identifies opportunities for improvement, and ultimately enhances customer satisfaction.
Project Management and Quality
Quality Assurance and Quality Control are integral components of project management frameworks, such as the Project Management Body of Knowledge (PMBOK) by the Project Management Institute (PMI).
Quality management is among the ten crucial knowledge areas in the Project Management Body of Knowledge (PMBOK) framework. It ensures that project deliverables meet or exceed customer requirements throughout the project lifecycle. This knowledge area encompasses three main processes:
Plan Quality Management:
- Identify quality requirements: This process involves understanding and documenting the quality expectations of stakeholders, including the customer, project team, and organization. Quality requirements can include specific criteria, standards, regulations, or customer preferences.
- Define quality standards: Based on the identified quality requirements, project managers establish quality standards that serve as benchmarks for evaluating project deliverables. These standards can be quantitative (e.g., defect rates, acceptance criteria) or qualitative (e.g., customer satisfaction, usability).
- Document the quality management plan: The project team develops a comprehensive plan outlining the strategies, processes, and resources required to achieve the desired quality levels. This plan includes quality control, quality assurance, and quality improvement activities.
Manage Quality:
- Implement quality control: Quality control activities focus on identifying and correcting defects in project deliverables. This process involves regular inspections, testing, and reviews to ensure the project's outputs meet the established quality standards.
- Implement quality assurance: Quality assurance activities focus on evaluating the effectiveness of the quality management system and processes. This includes audits, reviews, and assessments to ensure that the project follows the quality management plan and that any identified issues are addressed promptly.
- Manage quality records: Project teams maintain quality records, such as inspection reports, test results, and audit reports, to document the quality management activities and their outcomes. These records provide evidence of compliance with quality requirements and help in continuous improvement efforts.
Control Quality:
- Monitor quality: Project managers regularly monitor the quality of project deliverables and processes to assess their performance against the established quality standards. This includes tracking quality metrics, such as defect rates, customer satisfaction, and compliance with requirements.
- Analyze quality data: The collected quality data is analyzed to identify trends, patterns, and root causes of quality issues. This analysis helps understand the effectiveness of quality management activities and makes informed decisions for improvement.
- Recommend corrective and preventive actions: Based on the quality data analysis, project managers recommend corrective actions to address identified quality issues and preventive actions to minimize the likelihood of future problems. These actions may involve changes to processes, procedures, or training programs.
By integrating QA and QC into project management processes, organizations can ensure quality is built into every project lifecycle stage, from initiation to closing.
Beyond QA and QC
Quality Assurance and Quality Control are essential components of a broader Quality Management System (QMS), a formalized system that documents processes, procedures, and responsibilities for achieving quality objectives.
Popular QMS frameworks include:

ISO 9001:
- The International Organization for Standardization (ISO) published a set of standards for quality management systems (QMS).
- ISO 9001 is the world's most widely recognized and implemented QMS standard.
It provides a framework for organizations to establish, implement, and maintain a quality management system. - ISO 9001 certification demonstrates that an organization has a well-defined and effective QMS.
Total Quality Management (TQM):
- A management philosophy centered on achieving long-term success through customer satisfaction.
- TQM emphasizes continuous improvement, employee involvement, and a focus on quality in an organization's operations.
- TQM aims to create a culture of quality where everyone in the organization is committed to meeting or exceeding customer expectations.
Six Sigma:
- A data-driven methodology for improving processes and reducing defects.
- Six Sigma uses statistical methods to analyze and improve processes to achieve a defect rate of no more than 3.4 defects per million opportunities (DPMO).
- Six Sigma projects typically focus on reducing waste, improving efficiency, and increasing customer satisfaction.
Implementing a QMS helps organizations embed QA and QC practices into their daily operations, fostering a quality and continuous improvement culture.
Continuous Improvement and Quality
Continuous improvement is a fundamental principle of quality management, focusing on the ongoing effort to improve products, services, and processes over time. Some common continuous improvement methodologies include:

Plan-Do-Check-Act (PDCA) cycle:
- A continuous improvement framework involves planning, doing, checking, and acting.
- PDCA is an iterative process to improve quality, reduce waste, and increase efficiency.
- PDCA can be applied to any process or system, and it is a key part of Lean Six Sigma and Kaizen.
Lean Six Sigma:
- A methodology combining Lean and Six Sigma principles.
- Lean Six Sigma is a data-driven approach to improving quality and reducing waste.
- Lean Six Sigma is often used in manufacturing and healthcare but can be applied to any industry.
Kaizen:
- A Japanese philosophy of continuous improvement.
- Kaizen is based on the idea that small, incremental changes can lead to big results.
- Kaizen is often used in manufacturing but can be applied to any industry.
QA and QC play crucial roles in the continuous improvement process by providing data and insights on process performance, identifying areas for improvement, and verifying the effectiveness of implemented changes.
The Future of Quality in Business
Trends in Quality Assurance and Control
As businesses face increasing complexity, globalization, and customer expectations, QA and QC practices must evolve. Some emerging trends in quality management include:
- Digitalization and automation of quality processes
- Adoption of advanced technologies, such as AI and machine learning
- Shift towards predictive and prescriptive quality analytics
- Integration of quality into agile and DevOps methodologies
- Emphasis on supply chain quality management
Embracing these trends will enable organizations to streamline quality processes, detect and prevent defects more effectively, and respond quickly to changing market demands.
Advancing Technology and Quality
Technological advancements are transforming the way organizations approach quality management. Some examples include:
- Internet of Things (IoT) sensors for real-time process monitoring and control
- Big data analytics for identifying quality trends and predicting failures
- Augmented reality (AR) and virtual reality (VR) for immersive training and remote inspections
- Blockchain for ensuring traceability and authenticity of products
- 3D printing for rapid prototyping and customized quality solutions
By leveraging these technologies, organizations can enhance their QA and QC activities' efficiency, accuracy, and effectiveness, ultimately driving better quality outcomes.
Conclusion
In conclusion, Quality Assurance and Quality Control are two distinct but complementary aspects of quality management. QA proactively prevents defects through process-oriented activities, while QC focuses on reactively identifying and correcting defects through product-oriented activities.
Understanding the differences and synergies between QA and QC can help organizations develop robust quality management systems that deliver consistent, high-quality products and services to their customers. As businesses navigate an increasingly complex and competitive landscape, investing in effective QA and QC practices will drive growth, customer satisfaction, and long-term success.
FAQs

Get In Touch









